Can you use expired eye drops? Learn about serious health risks, bacterial contamination, and proper eye medication safety from medical professionals.

What happened when you use experired eye drops
Using expired eye drops poses significant health risks that can compromise your ocular safety and potentially lead to serious eye infections or complications. Eye drops, also known as ophthalmic solutions, are sterile pharmaceutical preparations designed for direct application to the delicate tissues of the eye, making their integrity and safety paramount for maintaining optimal eye health. These therapeutic solutions contain active pharmaceutical ingredients, preservatives, and stabilizing agents that deteriorate over time, rendering expired products potentially harmful rather than beneficial.
This comprehensive guide examines the critical safety concerns surrounding expired ophthalmic medications, explores the scientific rationale behind expiration dating, and provides evidence-based recommendations for proper eye drop usage and storage. We’ll cover the specific risks associated with using outdated ocular medications, explain why pharmaceutical expiry dates are non-negotiable for eye health, discuss different types of ophthalmic preparations and their unique considerations, and outline proper disposal methods and alternative solutions. Understanding these vital aspects of ophthalmic care will help you make informed decisions about your eye health while avoiding potentially dangerous complications.
Risks of Using Expired Eye Drops
Reduced Efficacy
Expired eye drops lose their therapeutic potency as active pharmaceutical ingredients undergo chemical degradation and molecular breakdown over time. The bioactive compounds in ophthalmic solutions, whether they’re antihistamines for allergic conjunctivitis, lubricants for dry eye syndrome, or antimicrobial agents for bacterial infections, experience molecular instability that compromises their clinical effectiveness. This degradation process accelerates when storage conditions deviate from manufacturer specifications, particularly when exposed to temperature fluctuations, direct sunlight, or humidity variations.
The pharmacokinetic properties of expired medications become unpredictable, potentially delivering subtherapeutic doses that fail to achieve desired clinical outcomes. For instance, expired antibiotic eye drops may not reach minimum inhibitory concentrations necessary to combat bacterial pathogens, potentially allowing infections to persist or worsen. Similarly, aged artificial tears may lose their viscosity and lubricating properties, providing inadequate relief for dry eye symptoms and potentially exacerbating ocular surface irritation.
Increased Risk of Infection
Bacterial contamination represents the most serious hazard associated with expired ophthalmic preparations, as preservative systems deteriorate and lose their antimicrobial efficacy over time. Benzalkonium chloride, chlorhexidine, and other commonly used preservatives in multi-dose eye drop formulations gradually lose their bacteriostatic and fungicidal properties, creating an environment conducive to microbial proliferation. This preservation breakdown allows opportunistic pathogens including Pseudomonas aeruginosa, Staphylococcus aureus, and various fungal species to colonize the solution.
Contaminated eye drops can introduce pathogenic microorganisms directly onto the corneal and conjunctival surfaces, potentially causing severe ocular infections such as bacterial keratitis, endophthalmitis, or orbital cellulitis. These conditions can result in permanent vision loss, corneal scarring, or even systemic complications requiring emergency medical intervention. The risk becomes particularly elevated in immunocompromised patients, contact lens wearers, or individuals with pre-existing ocular surface diseases.
Chemical Changes
Chemical degradation in expired ophthalmic solutions creates potentially toxic byproducts and alters the pH balance, osmolarity, and overall chemical composition of the preparation. Oxidation reactions, hydrolysis, and photodegradation can generate harmful metabolites that irritate delicate ocular tissues or cause allergic responses. The breakdown of stabilizing agents and buffering systems can shift the solution’s pH outside the physiologically compatible range, potentially causing chemical burns or severe ocular surface damage.
These chemical alterations may also affect the solution’s tonicity, creating hypertonic or hypotonic conditions that disrupt normal cellular function in corneal and conjunctival epithelia. Additionally, the degradation of chelating agents and antioxidants removes protective barriers against further chemical deterioration, accelerating the formation of irritating compounds and increasing the likelihood of adverse reactions.
Physical Changes
Observable physical alterations in expired eye drops serve as clear indicators of pharmaceutical degradation and potential safety hazards. Color changes, ranging from subtle yellow tinting to pronounced discoloration, typically indicate oxidation of active ingredients or preservative breakdown. Cloudiness, precipitation, or the formation of particulate matter suggests chemical instability, protein denaturation, or microbial contamination that renders the solution unsafe for ocular use.
Changes in viscosity, consistency, or the appearance of crystalline deposits indicate fundamental alterations in the formulation’s physical properties. These modifications can affect drug delivery, cause mechanical irritation to ocular surfaces, or indicate the presence of potentially harmful degradation products. Any visible changes in clarity, color, or consistency should be considered absolute contraindications to use, regardless of the stated expiration date.
Why Expiry Dates Matter

Where expired dates placed
The Science Behind Formulation Stability and Sterility
Pharmaceutical expiration dates represent the manufacturer’s guarantee of product safety, efficacy, and sterility based on rigorous stability testing and quality assurance protocols. These dates are established through accelerated aging studies, real-time stability assessments, and microbiological challenge tests that evaluate how environmental factors affect drug stability over time. The Food and Drug Administration (FDA) and other regulatory agencies require comprehensive documentation demonstrating that pharmaceutical products maintain their intended characteristics throughout their labeled shelf life.
Ophthalmic preparations undergo particularly stringent stability testing due to their direct application to vulnerable ocular tissues and the critical importance of maintaining sterility. Manufacturers conduct forced degradation studies, thermal stress testing, and photostability assessments to identify potential degradation pathways and establish appropriate storage conditions. These scientific evaluations determine the optimal formulation, packaging, and storage requirements necessary to ensure product integrity until the expiration date.
Role of Preservatives
Preservative systems in multi-dose eye drop formulations serve as the primary defense against microbial contamination during the product’s shelf life and after initial opening. These antimicrobial agents, including benzalkonium chloride, polyquaternium-1, and stabilized oxychloro complexes, maintain their efficacy through carefully balanced concentrations and synergistic interactions with other formulation components. The preservation system must remain effective against a broad spectrum of potential contaminants while maintaining compatibility with ocular tissues.
Over time, preservatives undergo chemical degradation, evaporation, or adsorption onto packaging materials, gradually reducing their antimicrobial capacity. This preservation failure creates opportunities for bacterial, fungal, or viral contamination that can occur even before visible signs of deterioration appear. The relationship between preservative efficacy and time is non-linear, with preservation capacity often declining rapidly after the expiration date, making expired products particularly susceptible to contamination.
Impact of Storage Conditions
Environmental factors significantly influence the stability and safety of ophthalmic preparations, with temperature, humidity, light exposure, and air quality all playing critical roles in determining product integrity. Storage temperatures outside the recommended range can accelerate chemical degradation, alter physical properties, or compromise preservative efficacy. High humidity environments promote hydrolysis reactions and can facilitate microbial growth, while excessive heat can denature proteins and destabilize emulsions.
Light exposure, particularly ultraviolet radiation, catalyzes photodegradation reactions that break down active ingredients and preservatives while generating potentially toxic photoproducts. Proper storage in cool, dry, dark environments helps maintain product stability, but cannot extend safety beyond the established expiration date. Even under optimal storage conditions, the complex interactions between formulation components, packaging materials, and environmental factors create inevitable degradation processes that progress predictably over time.
Types of Eye Drops & Expiry Considerations
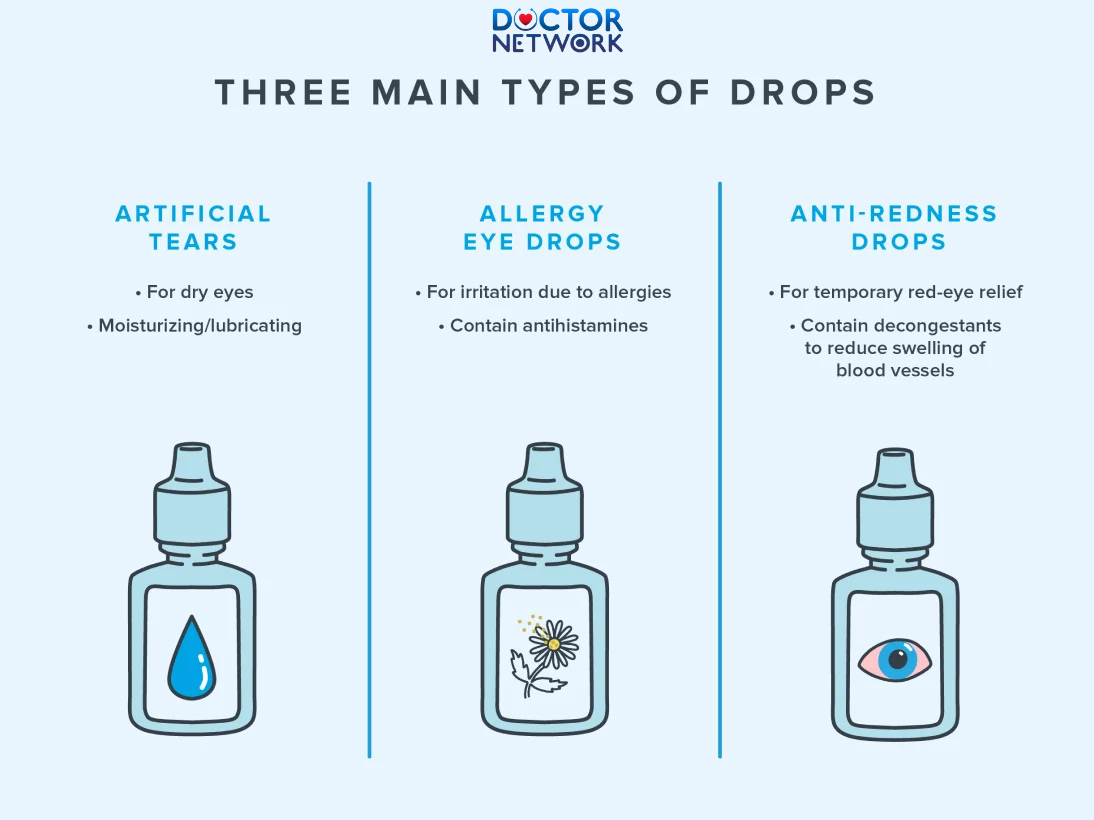
Some types of Eye Drops you need to know
Prescription vs. Over-the-Counter (OTC) Drops
Prescription ophthalmic medications typically contain higher concentrations of active pharmaceutical ingredients and may include potent compounds such as corticosteroids, antibiotics, or glaucoma medications that require precise dosing and timing. These prescription formulations often have shorter shelf lives due to the instability of their active components and the critical nature of maintaining therapeutic concentrations. Using expired prescription eye drops can result in treatment failure, disease progression, or serious complications that may require emergency medical intervention.
Over-the-counter eye drops, while generally considered safer due to their lower concentrations of active ingredients, still pose significant risks when used beyond their expiration dates. OTC artificial tears, allergy medications, and redness relievers undergo the same degradation processes as prescription medications, with preservative breakdown and potential contamination remaining primary concerns. The misconception that OTC products are inherently safer can lead to dangerous self-medication practices with expired formulations.
| Drop Type | Typical Shelf Life | Primary Risk When Expired | Special Considerations |
|---|---|---|---|
| Prescription Antibiotics | 1-2 years unopened | Treatment failure, resistance | Never use expired – can worsen infections |
| Prescription Steroids | 2-3 years unopened | Reduced anti-inflammatory effect | May contain preservatives harmful when degraded |
| OTC Artificial Tears | 2-4 years unopened | Contamination, irritation | Preservative-free versions expire faster |
| Allergy Drops | 2-3 years unopened | Reduced symptom control | Antihistamine degradation reduces efficacy |
| Glaucoma Medications | 1-2 years unopened | Vision loss progression | Critical for preventing blindness |
Preserved vs. Preservative-Free Drops
Preserved eye drops contain antimicrobial agents that extend shelf life and prevent contamination in multi-dose containers, but these preservatives can cause ocular surface toxicity with prolonged use or when degraded in expired products. Common preservatives like benzalkonium chloride become increasingly irritating as they break down, potentially causing more harm than benefit in expired formulations. The concentration and type of preservative system directly influence both the shelf life and the safety profile of expired products.
Preservative-free formulations, typically packaged in single-use vials or special multi-dose containers with filtration systems, eliminate preservative-related toxicity but have inherently shorter shelf lives and stricter storage requirements. These products rely entirely on sterile manufacturing and packaging to prevent contamination, making them particularly vulnerable to microbial growth once expired. Unit-dose vials must be discarded immediately after opening, and multi-dose preservative-free products typically expire within 30 days of first use, regardless of the printed expiration date.
Specific Types and Their Unique Considerations
Artificial Tears and Lubricating Drops Artificial tear formulations contain various polymers, electrolytes, and stabilizing agents that can undergo significant changes after expiration. Hyaluronic acid-based drops may lose their viscosity and lubricating properties, while carboxymethylcellulose solutions can develop bacterial contamination or changes in osmolarity that irritate rather than soothe dry eyes. Lipid-containing artificial tears are particularly susceptible to rancidity and oxidation that can cause severe ocular inflammation.
Antihistamine and Allergy Medications Expired antihistamine eye drops lose their ability to block histamine receptors effectively, potentially allowing allergic reactions to progress unchecked. Mast cell stabilizers like cromolyn sodium become less effective at preventing allergic responses, while combination products may have differential degradation rates for their various active ingredients. The resulting imbalanced formulations can cause unexpected side effects or reduced therapeutic benefit.
Antibiotic and Anti-inflammatory Preparations Expired antibiotic eye drops present particularly serious risks, as subtherapeutic concentrations may promote bacterial resistance while failing to treat infections effectively. Corticosteroid formulations lose their anti-inflammatory potency and may develop fungal contamination that can cause devastating mycotic infections. Combination antibiotic-steroid preparations face multiple degradation pathways that can create unpredictable and potentially dangerous chemical compositions.
What to Do Instead
Proper Storage of Eye Drops
Optimal storage conditions significantly extend the useful life of ophthalmic preparations while maintaining their safety and efficacy until the expiration date. Store all eye drops in cool, dry environments away from direct sunlight, with temperatures ideally maintained between 59-77°F (15-25°C) unless otherwise specified by the manufacturer. Bathroom medicine cabinets, despite their convenience, represent poor storage locations due to high humidity and temperature fluctuations from hot showers and baths.
Refrigeration may be required for certain formulations, particularly some prescription medications and preservative-free products, but standard artificial tears and most OTC preparations should be stored at room temperature. Avoid freezing eye drops, as ice crystal formation can permanently damage the formulation’s integrity and create particle contamination. Keep bottles tightly sealed when not in use to prevent evaporation, contamination, and oxidation, and never transfer drops to different containers that may not maintain sterility.
Checking Expiry Dates Before Use
Develop a systematic approach to monitoring expiration dates by regularly inspecting all ophthalmic medications in your possession and maintaining an inventory of current products. Check expiration dates before each use, not just when initially opening a new bottle, as this practice helps identify products that may have expired between uses. Mark the date of first opening on multi-dose containers, as many preservative-free products and some preserved formulations have shorter use periods after initial opening than their printed expiration dates suggest.
Create a rotation system that ensures older products are used before newer ones, and consider setting smartphone reminders or calendar alerts for products with approaching expiration dates. For individuals who use multiple eye medications, maintain a medication log that tracks expiration dates, opening dates, and usage frequency to prevent accidental use of expired products. This systematic approach becomes particularly important for patients managing chronic conditions like glaucoma or dry eye syndrome who may accumulate multiple bottles over time.
Eye Drop Safety Checklist:
- Check expiration date before every use
- Inspect solution for color changes, cloudiness, or particles
- Verify proper storage temperature and conditions
- Note the date when first opened on the bottle
- Replace preservative-free drops 30 days after opening
- Discard any drops that look or smell different
- Never share eye drops with others
- Wash hands thoroughly before application
- Avoid touching the dropper tip to any surface
Safe Disposal of Expired Drops
Proper disposal of expired eye drops protects both public health and environmental safety while preventing accidental ingestion or misuse by children, pets, or other household members. Do not flush expired eye drops down toilets or drains, as pharmaceutical compounds can contaminate water supplies and harm aquatic ecosystems. Instead, remove or obscure all identifying information from the packaging and dispose of the entire product in household trash, preferably mixed with unpalatable substances like coffee grounds or cat litter.
Many communities offer pharmaceutical take-back programs through local pharmacies, hospitals, or law enforcement agencies that provide safe disposal services for expired medications. The Drug Enforcement Administration (DEA) sponsors National Prescription Drug Take Back Days twice yearly, providing convenient disposal opportunities for all types of medications including eye drops. Contact your local pharmacy or healthcare provider for information about ongoing take-back programs in your area.
When to Purchase New Drops
Replace expired eye drops immediately upon discovery, regardless of remaining volume or apparent condition, as the risks associated with expired ophthalmic products far outweigh any economic considerations. For individuals with chronic conditions requiring regular eye drop therapy, establish a purchasing schedule that ensures continuous access to fresh medications without accumulating excessive inventory. Consider the frequency of use when purchasing multiple bottles, as buying too many at once can lead to waste if products expire before use.
Consult with your healthcare provider or pharmacist about appropriate quantities to purchase based on your usage patterns and the stability characteristics of your specific medications. For emergency situations or travel, maintain a small reserve of fresh artificial tears or prescribed medications, but ensure these backup supplies are rotated regularly to prevent expiration. Consider the cost-effectiveness of different package sizes, but prioritize safety over savings when making purchasing decisions.
When to Seek Professional Advice
Recognizing Symptoms Requiring Ophthalmologic Care
Seek immediate attention from an ophthalmologist or emergency medical services if you experience severe eye pain, sudden vision loss, flashing lights, or curtain-like visual field defects after using any eye drops, expired or otherwise. These symptoms may indicate serious conditions such as acute angle-closure glaucoma, retinal detachment, or severe chemical injury that require urgent intervention to prevent permanent vision loss. Additionally, signs of serious infection including increasing redness, purulent discharge, swelling of the eyelids or surrounding tissues, or fever warrant immediate professional evaluation.
Contact your eye care provider promptly if you develop persistent irritation, burning, stinging, or foreign body sensation after using eye drops, particularly if symptoms worsen rather than improve with time. Changes in vision quality, including blurriness, halos around lights, or difficulty with night vision, may indicate adverse reactions to expired medications or underlying eye conditions that require professional diagnosis and treatment. Never attempt to self-treat serious eye symptoms with over-the-counter products, expired or current.
The Importance of Professional Consultation
Regular comprehensive eye examinations by qualified optometrists or ophthalmologists provide essential monitoring for individuals who use eye drops regularly, whether for chronic conditions or occasional symptoms. These professionals can assess the appropriateness of your current medications, monitor for side effects or complications, and adjust treatment plans based on changing needs or new therapeutic options. Professional guidance becomes particularly important for individuals with multiple eye conditions, those taking systemic medications that may interact with eye drops, or patients with compromised immune systems.
Healthcare professionals can provide personalized recommendations for proper eye drop selection, usage techniques, and storage practices based on your specific needs and medical history. They can also identify early signs of medication-related complications, screen for underlying conditions that may be causing persistent symptoms, and ensure that your treatment approach addresses root causes rather than just managing symptoms. Establishing a relationship with an eye care professional provides valuable continuity of care and professional oversight for your long-term ocular health.
| Symptom Category | Specific Signs | Urgency Level | Recommended Action |
|---|---|---|---|
| Vision Threatening | Sudden vision loss, severe pain, flashing lights | Emergency (0-2 hours) | Go to ER or call ophthalmologist immediately |
| Infection Signs | Purulent discharge, increasing redness, swelling | Urgent (same day) | Contact eye doctor or urgent care |
| Irritation/Allergy | Mild redness, tearing, itching | Routine (1-3 days) | Schedule appointment with eye care provider |
| Medication Questions | Dosing, interactions, side effects | Non-urgent | Consult pharmacist or eye doctor at next visit |
Final Medical Recommendation: Zero Tolerance for Expired Drops
The evidence overwhelmingly demonstrates that using expired eye drops presents unacceptable risks to ocular health and overall well-being, with potential consequences ranging from treatment failure to serious vision-threatening infections. The complex degradation processes affecting pharmaceutical formulations after expiration compromise both safety and efficacy, creating products that may cause more harm than benefit. Chemical breakdown of active ingredients, preservative failure, and potential contamination make expired ophthalmic preparations fundamentally unsuitable for therapeutic use, regardless of their apparent condition or remaining volume.
Your vision represents one of your most precious senses, deserving protection through evidence-based medical practices rather than convenience-driven compromises. The relatively modest cost of replacing expired eye drops pales in comparison to the potential expenses and consequences associated with treating complications from contaminated or degraded products. Serious ocular infections, allergic reactions, or treatment failures resulting from expired medications can require extensive medical intervention, cause permanent vision damage, and significantly impact quality of life.
Final recommendation: Never use expired eye drops under any circumstances. Instead, prioritize proper storage practices, regular inventory management, and appropriate disposal methods to maintain a safe supply of effective ophthalmic medications. When in doubt about any aspect of eye drop safety, storage, or usage, consult qualified healthcare professionals who can provide personalized guidance based on current medical evidence and your individual needs. Your commitment to using only current, properly stored eye drops represents a simple but crucial investment in preserving your vision and maintaining optimal eye health throughout your lifetime.
Frequently common questions about using expired eye drops along with detailed answers
1. Can you use expired eye drops safely?
No, it is not recommended to use expired eye drops. After the expiration date, the active ingredients may degrade, reducing their effectiveness. Also, preservatives that prevent bacterial growth can break down, increasing the risk of contamination and eye infections.
2. What are the risks of using expired eye drops?
Using expired eye drops can cause several issues:
Reduced effectiveness, meaning your eye condition may not improve or could worsen.
Risk of bacterial contamination leading to infections such as redness, discharge, and swelling.
Possible allergic reactions or irritation like itching, redness, or burning.
Potential chemical changes that may harm eye tissues.
3. What should I do if I accidentally use expired eye drops?
If you accidentally use expired eye drops, stop using them immediately and discard the bottle. Monitor your eyes for any signs of irritation, redness, pain, or discharge. If any symptoms of infection or allergic reaction occur, consult an eye care professional promptly.
4. Why do eye drops have expiration dates?
Expiration dates indicate the period during which the manufacturer guarantees the safety and effectiveness of the product. Beyond this date, the chemical composition can change, preservatives may lose potency, and contamination risk increases, all of which compromise safety and efficacy.
5. How can I safely use and store eye drops?
Always check the expiration date before use and do not use expired products.
Store eye drops in a cool, dry place away from direct sunlight as per the instructions.
Avoid touching the dropper tip to your eye or any surface to prevent contamination.
Follow dosage instructions carefully and maintain good hygiene by washing hands before application.
References
U.S. Food and Drug Administration (FDA) – Don’t Be Tempted to Use Expired Medicines:
https://www.fda.gov/drugs/special-features/dont-be-tempted-use-expired-medicines
(This link provides a clear directive from a major regulatory body against using any expired medicines, including eye drops, due to risks like bacterial growth and reduced effectiveness.)American Academy of Ophthalmology (AAO) – How to Use Eye Drops Correctly:
https://www.aao.org/eye-health/tips-prevention/how-to-use-eye-drops
(This link from a leading ophthalmological organization specifically advises checking expiration dates for eye drops and discarding them if expired.)Scientific Study (Example of contamination risk) – Microbial contamination of in-use ocular medications (Tsegaw A, et al., 2017):
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5426132/
(This study demonstrates the real-world risk of microbial contamination in eye drops, a risk that significantly increases when preservatives in expired drops fail.)
Kiểm Duyệt Nội Dung
More than 10 years of marketing communications experience in the medical and health field.
Successfully deployed marketing communication activities, content development and social networking channels for hospital partners, clinics, doctors and medical professionals across the country.
More than 6 years of experience in organizing and producing leading prestigious medical programs in Vietnam, in collaboration with Ho Chi Minh City Television (HTV). Typical programs include Nhật Ký Blouse Trắng, Bác Sĩ Nói Gì, Alo Bác Sĩ Nghe, Nhật Ký Hạnh Phúc, Vui Khỏe Cùng Con, Bác Sỹ Mẹ, v.v.
Comprehensive cooperation with hundreds of hospitals and clinics, thousands of doctors and medical experts to join hands in building a medical content and service platform on the Doctor Network application.